Generic Name:
donepezil hydrochloride
Trade Name:
Aricept ® [Eisai (also marketed by Pfizer)]
IUPAC Name:
2-[(1-benzyl-4-piperidyl)
methyl]-5,6-dimethoxy-2,3-
dihydroinden-1-one hydrochloride
Dosage Forms/Routes:
Tablet/oral;
Solution/oral;
Tablet (orally disintegrating)/oral
Major Metabolites:
6-O-desmethyl donepezil;
donepezil-cis-N-oxide;
5-O-desmethyl donepezil;
5-O-desmethyl donepezil glucuronide
Database Search Links:
[PubMed]
[Search PubMed for
Randomized Controlled Trials]
[PubChem]
[PDSP Ki database] |
Initial Approval:
11/25/1996
Manufacturers:
[FDA Search]
Product Insert:
[Link]
RxList:
[Link]
Epocrates:
[Link]
Empirical Formula:
C24H30ClNO3
Molecular Mass:
415.953 g/mol |
Indications:
Treatment of mild to moderate dementia of the Alzheimer's type
|
Possible Mechanisms of Action:
Donepezil is a selective and reversible acetylcholinesterase inhibitor (1). Donepezil is a mixed type inhibitor of acetycholinesterase, meaning that it exhibits both noncompetitive and competitive components of inhibition (1). |
Chemical Class:
benzylpiperidine derivative
PubChem 2D Structure:

"3D" Structure (Requires Chime):
[Link]
|
Generic Name:
galantamine hydrobromide
Trade Name:
Razadyne ® [Janssen]
Common Chemical Name:
(4aS,6R,8aS)-4a,5,9,10,11,12-
hexahydro-3-methoxy-11-
methyl-6H-benzofuro
[3a,3,2-ef][2]benzazepin-6-ol
hydrobromide
Major Metabolites:
O-desmethylgalantamine glucuronide;
N-desmethylgalantamine;
epigalantamine
Dosage Forms/Routes:
Tablet/oral;
Solution/oral;
Capsule (extended release)/oral
Database Search Links:
[PubMed]
[Search PubMed for
Randomized Controlled Trials]
[PubChem]
[PDSP Ki database] |
Initial Approval:
02/28/2001
Manufacturers:
[FDA Search]
Product Insert:
[Link]
[Link] (ER)
RxList:
[Link]
Epocrates:
[Link]
Empirical Formula:
C17H22BrNO3
Molecular Mass:
368.266 g/mol
|
Indications:
Treatment of mild to moderate dementia of the Alzheimer's type |
Possible Mechanisms of Action:
Galantamine is a competitive and reversible inhibitor of acetylcholinesterase (2).
The drug also acts as a positive allosteric modulator at nicotinic acetylcholine
receptors including the alpha4beta2 subtype (3). |
Chemical Class:
phenanthrene alkaloid
PubChem 2D Structure:
 |
Generic Name:
memantine hydrochloride
Trade Name:
Namenda ® [Forest Labs]
IUPAC Name:
3,5-dimethyladamantan-1-
amine hydrochloride
Major Metabolites:
N-gludantan conjugate of memantine;
6-hydroxymemantine;
1-nitroso-deaminated memantine
Dosage Forms/Routes:
Tablet/oral;
Solution/oral
Database Search Links:
[PubMed]
[Search PubMed for
Randomized Controlled Trials]
[PubChem]
[PDSP Ki database] |
Initial Approval:
10/16/2003
Manufacturers:
[FDA Search]
Product Insert:
[Link]
RxList:
[Link]
Epocrates:
[Link]
Empirical Formula:
C12H22ClN
Molecular Mass:
215.763 g/mol |
Indications:
Treatment of moderate to severe dementia of the Alzheimer's type |
Possible Mechanisms of Action:
Memantine
is a moderate affinity, voltage-dependent, noncompetitive NMDA receptor antagonist (4). Memantine is also a voltage-dependent, reversible, noncompetitive antagonist at serotonin 5-HT3 receptors (5, 6). In addition, memantine acts as a noncompetitive open channel blocker at some subtypes of neuronal nicotinic acetylcholine receptors,
including alpha4beta2 subunit-containing receptors (7) and alpha7 subunit-containing receptors (10).
|
Chemical Class:
cyclic amine
PubChem 2D Structure:
 |
Generic Name:
rivastigmine tartrate
Trade Name:
Exelon ® [Novartis]
IUPAC Name:
[3-(1-dimethylaminoethyl)
phenyl] (ethyl-methyl-
amino)methanoate; 2,3-
dihydroxybutanedioic acid
Major Metabolite:
NAP 226-90
Dosage Forms/Routes:
Capsule/oral;
Solution/oral
Database Search Links:
[PubMed]
[Search PubMed for
Randomized Controlled Trials]
[PubChem]
[PDSP Ki database] |
Initial Approval:
04/21/2000
Manufacturers:
[FDA Search]
Product Insert:
[Link]
RxList:
[Link]
Epocrates:
[Link]
Empirical Formula:
C18H28N2O8
Molecular Mass:
400.424 g/mol |
Indications:
Treatment of mild to moderate dementia of the Alzheimer's type |
Possible Mechanisms of Action:
Rivastigmine is a slowly reversible (pseudo-irreversible) inhibitor of acetylcholinesterase and butyrylcholinesterase (8). Rivastigmine inhibits acetylcholinesterase in a noncompetitive manner (9). |
Chemical Class:
carbamate derivative
PubChem 2D Structure:
 |
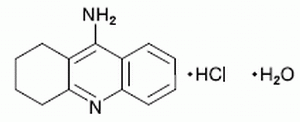
Generic Name:
tacrine hydrochloride
Trade Name:
Cognex ® [First Horizon]
Common Chemical Name:
1,2,3,4-tetrahydro-9-
acridinamine monohydrochloride monohydrate
Major Metabolites:
1-hydroxytacrine
Dosage Forms/Routes:
Capsule/oral
Database Search Links:
[PubMed]
[Search PubMed for
Randomized Controlled Trials]
[PubChem]
[PDSP Ki database] |
Initial Approval:
09/09/1993
Manufacturers:
[FDA Search]
Product Insert:
[Link]
RxList:
[Link]
Epocrates:
[Link]
Empirical Formula:
C13H14N2•HCl•H2O
Molecular Mass:
252.74 g/mol
|
Indications:
Treatment of mild to moderate dementia of the Alzheimer's type |
Possible Mechanisms of Action:
Tacrine is a reversible acetylcholinesterase inhibitor (1). Tacrine is a mixed type inhibitor of acetycholinesterase, meaning that it exhibits both noncompetitive and competitive components of inhibition (1). Tacrine is also a reversible inhibitor of butyrylcholinesterase (1). |
Chemical Class:
acridine derivative
2D Structure:
 |




























